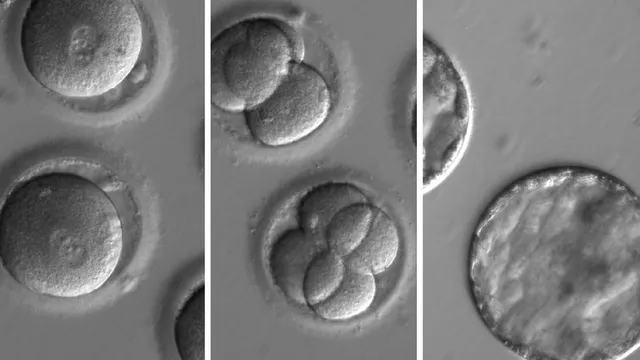
A revolution in gene editing is underway - and at the forefront is David Liu, an American molecular biologist whose pioneering work is rewriting the building blocks of life with unprecedented precision.
A professor at the Broad Institute of the Massachusetts Institute of Technology and Harvard, Liu was awarded a Breakthrough Prize, or Oscar, in the natural sciences for developing two transformative technologies: one is already improving the lives of patients with severe genetic diseases, and the other is poised to change medicine in the years to come.
He spoke to AFP ahead of a ceremony in Los Angeles to present the prestigious award, which was established in Silicon Valley.
He will receive $3 million for his work on "basic editing" and "primary editing" and plans to donate most of the prize to support his charitable foundation.
"The ability to change the DNA sequence of our choice into a new sequence of our choice is fundamentally a very powerful ability," the 51-year-old said, envisioning applications not only in human medicine but also in fields such as developing more nutritious or disease-resistant crops.
Correcting the code
DNA consists of four chemical "letters" - the nucleotide bases A, G, T and C. Mutations in this sequence cause thousands of human diseases, but until recently gene editing could only correct a limited number of them.
Even CRISPR-Cas9, the groundbreaking technology that won a Nobel Prize in 2020, has serious limitations.
This technology cuts both strands of the DNA helix, making it most useful for disrupting rather than correcting genes, while the process can introduce new errors.
"The ability to use genome editing to treat genetic diseases requires, in most cases, ways to correct misspelled DNA, not just to disrupt a gene," Liu said.
That insight prompted his lab to develop a basic edit that uses the Cas9 protein - disabled so it can no longer cut both DNA strands - to find a target DNA sequence and another enzyme to convert one letter to another - for example, C to T or G to A.
The reverse change - from T to C or A to G - was more difficult. Liu's team overcame the challenge by creating entirely new enzymes.
These basic editors can now correct about 30 percent of the mutations that cause genetic diseases. The technology has now passed at least 14 clinical trials.
In one of them, Beam Therapeutics-which Liu co-founded-announced that it had treated patients with AATD, a rare genetic disease affecting the lungs and liver, with a single drug administration.
While traditional gene therapies often destroy defective genes or bypass them, base editing fixes the mutation itself.
"This was the first time people corrected a mutation that causes a genetic disease in a patient," Liu said.
Hope for cystic fibrosis
Basic editing, quickly dubbed "CRISPR 2.0," cannot correct every mutation.
About 70 percent of the roughly 100,000 known disease-causing mutations remain beyond its reach, including those caused by missing or redundant letters.
To expand the toolbox, Liu's lab introduced primary editing in 2019, a method capable of replacing entire stretches of faulty DNA with corrected sequences.
If CRISPR is like a pair of scissors cutting DNA, and basic editing is like using a pencil to correct individual letters, then primary editing is the equivalent of the "find and replace" feature in the Word text editor.
Creating this tool required a series of breakthroughs that Liu's team describes as "small miracles." The result, he said, is "the most versatile way we know of editing the human genome."
Among the targets Liu and his team have already pursued with basic editing is cystic fibrosis, a common genetic disease usually caused by three missing letters in the DNA that causes a build-up of thick mucus in the lungs and digestive system.
Liu's lab makes much of its work freely available, sharing blueprints of DNA through a nonprofit library used by tens of thousands of labs around the world.
"The science we create - which is ultimately funded by the public, through governments and donors - ultimately pays back to benefit society."
This year's breakthrough awards come at a difficult time for American science, as President Donald Trump's administration cuts funding to institutions such as the National Institutes of Health (NIH).
"The National Institutes of Health is a treasure not only for this country, but for the world," Liu said. "Trying to destroy the heart of what supports science in this country is like trying to burn the seed for planting." I BGNES

 Breaking news
Breaking news
 Europe
Europe
 Bulgaria
Bulgaria